Multiscale computer model of the spinal dorsal horn.
Abstract
Background and Aims: Discovery of improved chronic pain treatments has proven difficult due to our incomplete understanding of sensory processing mechanisms. Pain-related sensory input from primary afferents is processed in the spinal dorsal horn (SDH) before being relayed to the brain. Processing of these signals by local spinal interneurons profoundly influences whether tactile stimuli are correctly or incorrectly perceived as painful. Specifically, a reduction in synaptic inhibition, or disinhibition, following nerve injury opens the gate between tactile input and nociceptive projection neurons such that innocuous touch becomes painful [1]. Beyond its role in gating the nociceptive circuit, inhibition in the SDH also organizes neuronal receptive fields. Pathological disruption of receptive fields alters the spatial processing of touch and may underlie the clinical observations that diffuse/dynamic stimuli are more distressing to pain patients than punctate/static stimuli [2,3]. Although recent studies have identified different types of spinal interneurons and begun to disentangle their synaptic connectivity, it remains unclear how the SDH circuit processes tactile input or how that processing is disrupted under neuropathic pain conditions.
Methods: To explore sensory processing in the SDH, we developed a computational model of the SDH circuit that is tightly constrained by experimental data [4]. Our model comprises conductance-based neuron models that reproduce the characteristic firing patterns of spinal neurons. Different spinal neuron populations and microcircuits were synaptically connected according to available experimental data [5,6]. Specifically, receptive fields of spinal neurons were developed with an excitatory center-inhibitory surround structure [7] to account for spatial processing of touch input. Using a genetic algorithm (GA), synaptic weights between pre- and post-synaptic neuron populations were optimized to reproduce spinal projection neuron firing rates (model output) in response to primary afferent firing rates (model input) across a range of tactile stimulus intensities. Neuropathic pain conditions were simulated via reductions in synaptic inhibition to examine their effect on SDH circuit function.
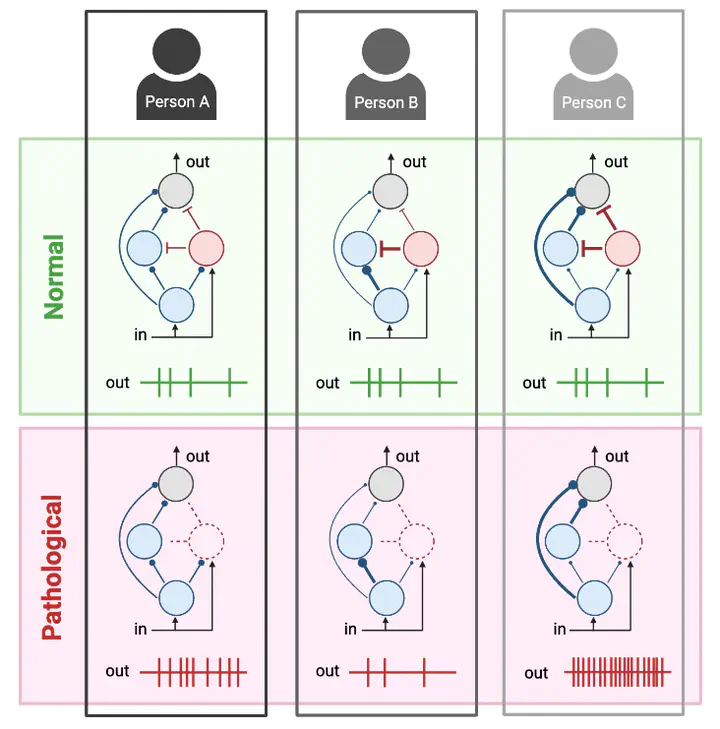
Results: Model optimization revealed that distinct synaptic weight combinations could produce equivalent SDH circuit responses to normal tactile stimulation – an example of degeneracy – but these circuits responded differently to pathological perturbation (e.g. disinhibition). In other words, differently organized circuits may function equivalently under normal conditions but fail differently under pathological conditions, which may explain why two individuals respond differently to pathological insult or therapeutic intervention. Further analysis of different SDH circuits exposed compensatory mechanisms between different model parameters, another hallmark of degenerate systems. Our top SDH model was validated by confirming that it responded to disinhibition and ablation of specific spinal neuron types in a manner consistent with experiments. Simulating reduced synaptic inhibition in the SDH model revealed that subtle differences in disinhibition at the cellular level may translate to large differences in SDH output due to compounding effects at the circuit level. Disinhibition also reshaped receptive field organization in ways that increased spatial summation of tactile input. Finally, our model made testable predictions regarding multiscale targets for combating the effects of disinhibition.
Conclusions: Our data-driven, multiscale model offers a valuable resource for interpreting experimental results and testing hypotheses in silico to plan experiments for examining normal and pathological SDH circuit function. The model reveals degeneracy in the SDH that may have important clinical implications for guiding the development of new treatments to help patients afflicted with neuropathic pain.