A novel computational model underlying the spiking dynamics of a subfornical organ neuron.
Abstract
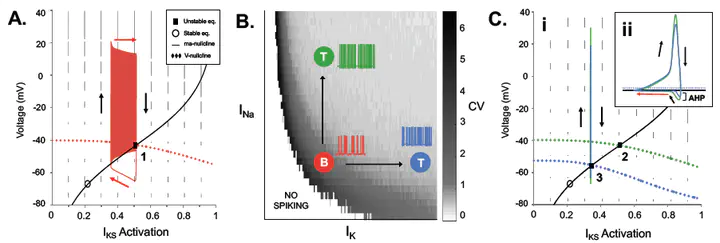
The subfornical organ (SFO), a circumventricular organ that lacks the blood brain barrier (BBB), plays an important role in sensing various blood-borne signals from the peripheral circulation. SFO neurons then integrate these signals and project them across the BBB to regulate critical autonomic functions, including cardiovascular and energy homeostasis. Previous findings from in vitro studies have established that SFO neurons exhibit an immense heterogeneity in their expression of ionic currents and spiking behaviour, as well as their response to different circulating peptides. Insight into the mechanisms behind this heterogeneity is critical for understanding how the SFO integrates and regulates autonomic function, but currently the mechanisms are poorly understood due to the limitations of patch-clamp techniques. To address this limitation, we built a Hodgkin-Huxley style (HH) single neuron model of an SFO neuron using parameter search techniques to match in vitro spike train data. The resulting HH model demonstrated the two major spiking behaviours exhibited by different SFO populations, tonic firing and burst firing, where the burst behaviour is characterized by robust membrane potential bistability. This was accomplished through the addition of a non-selective cation current, transient potassium current, persistent sodium current, and current noise. Established methods for neuronal spike train analysis were then used to classify SFO neurons based on their spiking behaviour and membrane properties. For example, the coefficient of variation and histogram distribution analyzed membrane potential variability and modality, respectively. Dynamic systems analysis further characterized the neuronal mechanisms supporting the differences between tonic and burst firing neurons. These methods were further used to predict the behaviour of SFO neurons in response to the binding of Angiotensin-II (ANG-II), a peptide hormone that acts within the SFO to influence various functions including blood pressure and fluid balance. Future applications of this model would allow us to study the integration of ANG-II and other autonomic signals within the SFO; a method that is limited using current techniques.